Abstract
Background: Cytokine release syndrome (CRS) is a well described, non-specific toxicity of CAR therapy caused by high-level immune activation. Generally, CRS starts within a week of CAR-T cell infusion, is typically self-limited, and mitigation strategies utilizing tocilizumab (anti-IL6 receptor antibody) and/or corticosteroids are rapidly effective. Despite initial improvement, delayed manifestations including disseminated intravascular coagulation (DIC) and macrophage activation syndrome (MAS) may occur in some for whom CAR mediated inflammatory responses continue to evolve. To improve upon the safety profile of CAR therapy, we report on our experience with DIC and MAS.
Design: We report on the toxicity profile of the first 30 children and young adults with relapsed/refractory CD22+ ALL treated on our phase I dose escalation anti-CD22 CAR therapy. All had routine monitoring of cytokines, fibrinogen, D-Dimer, PT/INR, PTT and CBC. Following observations of clinically significant DIC, we prospectively evaluated markers of activated coagulation, including: thrombomodulin, E-selectin, s-VCAM, v-ICAM, P-selectin, prothrombin fragment 1 + 2, tissue factor, and PAI-1. Markers for MAS, including ferritin, triglycerides and soluble IL2R were monitored, as well as immunohistochemical staining for hemophagocytosis on the bone marrow aspirate/biopsy.
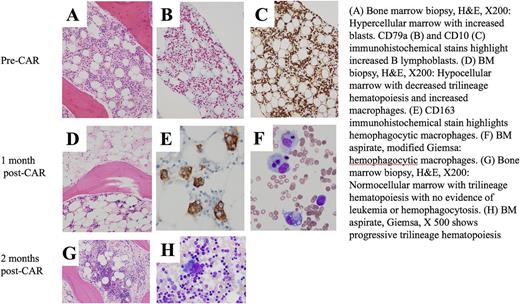
Results: From December 2014 to July 2017, 30 patients with ALL were treated at 3 dose levels. CRS was seen in 24/30 (80%), which was grade 1-2 in all, except for one grade 4. Eight of 24 (33%) with CRS developed DIC, which was grade 2 (no bleeding) in 3 patients and grade 3 (with bleeding) in 5 patients. Bleeding complications were primarily mucosal and included diffuse alveolar hemorrhage, epistaxis, hematuria, subconjunctival hemorrhage and petechiae/purpura that appeared disproportionate to the platelet count and typically occurred after resolution of acute CRS. Correspondingly, morphologic evaluation revealed hypogranular/agranular platelets seen in 16 of 20 patients (confirmed by electron microscopy in one), of whom 7 had DIC, suggesting an acquired platelet function defect; such patients received higher transfusion support. In those with DIC, PT/INR/PTT and D-Dimer rose with onset of CRS. Fibrinogen, initially elevated with the onset of CRS, fell precipitously with resolution of fever. At the time of clinically evident DIC, in 8 of 12 evaluable subjects, P-selectin values rose at least 50%, on average 17.6 days after CAR-T infusion and in 11 of 12 evaluable subjects, endothelial markers rose and peaked 2 weeks after CAR-T infusion (mean 15.2 days). Treatment of DIC was primarily supportive; methylprednisolone, starting at 0.5-1 mg/kg was initiated in patients with active bleeding, and continued q6-8 hours until stabilization. Resolution of DIC typically coincided with decrease in ferritin, suggesting an inflammatory mediated coagulopathy. Accordingly, patients with DIC also had statistically significantly higher ferritin (median 361,585 v 14,349, p<0.0001) IFN-g, IL-1b, IL-6, IL-8, IL-10 and MIP-1a (p<0.01 for all) than in those without DIC, mimicking elevations seen in MAS. Further supporting the link to MAS, 3 of 4 patients with DIC had soluble IL2R levels > 5-fold x the upper limit of normal at 2 weeks post CAR therapy. Additionally, one patient with DIC had active hemophagocytosis at the 1 month evaluation (Figure), which in the constellation of hepatic transaminitis, cytopenias and hyperferritinemia led to a diagnosis of MAS. Treatment with single agent anakinra, an IL-1 receptor antagonist, initiated as a steroid-sparing agent, was well tolerated and led to full resolution of hemophagocytosis; importantly, it did not impede ongoing CAR response, with clearance of all minimal residual disease from the one month to the two-month restaging time point.
Conclusion: An intricate relationship exists between the initial inflammatory CRS, and later complications of DIC and MAS potentially induced by CAR T related inflammation. Although steroids and supportive care measures may stabilize DIC, cytokine profiles, which include elevated IL-1b, can assist when choosing targeted, steroid-sparing therapies such as anakinra. Future directions will include exploring the link between inflammation, DIC, and MAS and optimizing targeted anti-inflammatory strategies earlier in the treatment course to prevent serious complications.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal